
Wired for the future
Executive summary
Game-changing developments are taking place in the emerging field of neurotechnology, with ever growing levels of ambition. The recent announcement of Open AI co-funding the new Brain-Computer Interface start-up Merge Labs, with the expected valuation of USD 850 mln, has made clear that the brain space is becoming a centre-stage of the global technological rivalry. Nudge, the company building non-invasive brain interface using focused ultrasound, has just raised USD 100 mln. Meanwhile in Europe, two milestones have been reached in recent weeks, with CorTec emerging as Germany’s first implantable Brain-Computer Interface developer, announcing the first human implantation of its system, and INBRAIN reporting the initial results of their first-in-human study with a graphene-based BCI during tumour resection surgery.
The case for investing in neurotechnology is compelling given its significant potential to address unmet medical needs and complement pharmacological approaches in managing the growing burden of brain disorders. New frontiers of application are constantly being crossed. Further, when integrated with wearable sensor technologies, neurotechnology holds great promise for developing innovative platforms for prevention and early diagnosis. These platforms can leverage currently underused data and build on new biomarkers and emerging knowledge of modifiable risk factors. They can enable a shift from reactive approaches, focused on treating the already developed disorders, to proactive ones, focused on prevention.
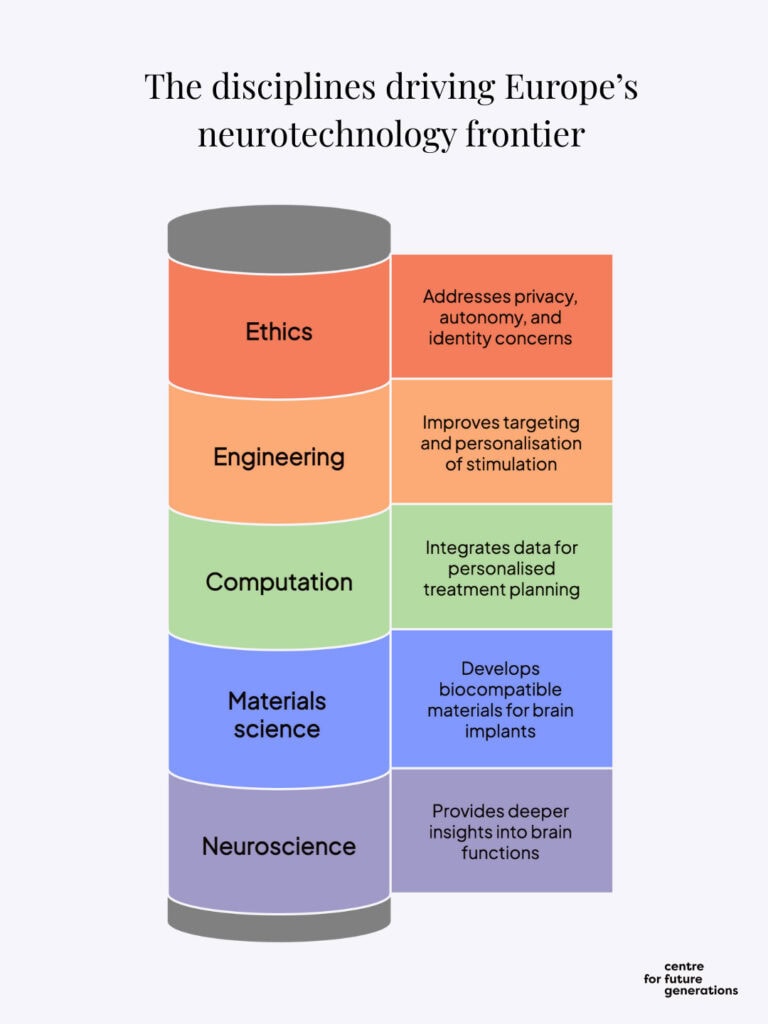
It is also essential to recognise the inherently interdisciplinary nature of the field: investing in neurotechnology entails supporting neuroscience, computer science, advanced materials, and many other related disciplines. This interdisciplinary nature strengthens the rationale for establishing a precompetitive platform where research groups, startups and industry could collaborate on foundational developments before going to the market.
To help guide and optimise investments in Europe, the EU should establish a Neurotechnology Funding Board, a new mechanism to make existing financial instruments better suited and adapted to the needs of neurotechnology and develop domain-specific ones to meet unique needs of this evolving field.
Finally, pooling reimbursement schemes across a group of EU Member States would allow innovative neurotechnology companies to scale, hence ensuring broad access across the affected population.
Given the emerging role of neurotechnology as an important component of brain health as well as locus of interdisciplinary innovation, Europe needs to become significantly more strategic in its approach to the field, making it one of its areas of excellence and competitive strength.
The rise of neurotechnology in healthcare
Neurotechnology comprises devices and tools that interact with the brain or the nervous system, to either monitor or influence neural activity. These devices can be implanted within the brain or body or worn externally and act through the skin or skull. Neurotechnology should be seen as an essential component of brain health and a critical complement to pharmacological and psycho-social interventions. Its applicability will continue to grow in the face of the rising prevalence of neurological disorders and the mental-health epidemic, which shows few signs of subsiding[1].
Approximately 165 million Europeans, or one-third of Europe’s population, are living with a brain condition, a term that encompasses a wide range of neurological and mental-health conditions.[2] Neurological disorders are a leading cause of illness and disability. In the EU, they ranked third in 2017, after cardiovascular diseases and cancers, accounting for 13.3% of total disability-adjusted life years and 19.5% of total deaths.[3] The economic burden is equally significant. The annual cost of brain disorders in Europe was estimated in 2018 at €798 billion, including health-care expenses, lost productivity and informal care.[4]
By 2050, brain disorders are projected to increase globally by 22% from 2021 estimates. While the human burden of disease is overwhelming, economic consequences will rise exponentially as well. Dementia spending is expected to represent 11% of all healthcare spending in Germany by 2050, with the baseline attributable dementia spending reaching USD 56.5 bln in 2050, as opposed to USD 13.5 bln in 2021, while USD 75.8 bln in 2050 in the scenario in which diagnosis and treatment rates accelerate, USD 85.5 bln if nursing home-based rates accelerate, and USD 86.5 bln if unit costs per patient accelerate (See Figure 1).
Many disorders of the nervous system and mental-health conditions lack effective pharmaceutical therapy, or cannot be treated with medication, while neurotechnology solutions can enhance neuroplasticity and motor recovery. Neurofeedback can help retrain brain circuits involved in fear and memory, aiding patients who suffer from post-traumatic stress disorder. Cochlear implants restore hearing in severe sensorineural deafness, whereas no drug or therapy can regrow damaged cells in the cochlea.
These examples show how neurotechnology allows for the emergence of approaches that can repair, restore or replace lost or impaired function.
New developments in neurotechnology medicine open the prospect of treating disorders of mood, sensation, learning, memory and cognition. Many solutions are no longer theoretical, but increasingly ripe for application, as evidenced by the recent approval by the US Food and Drug Administration (FDA) of a first-of-its-kind neuroimmune modulation device SetPointSystem for the treatment of adults living with moderate-to-severe rheumatoid arthritis.
Multimodal approaches that combine different therapies can help shift treatment of chronic pain away from reliance on medication, including opioids. Studies have shown that patients who undergo spinal cord stimulation therapy have been able to reduce their average daily systemic opioid dose by at least half.[5] While pharmaceuticals have multiple tissue barriers to cross to act on their target, neurotechnology solutions form a direct link. Their effect can be immediate and localised to the specific target, reducing the number of side-effects.
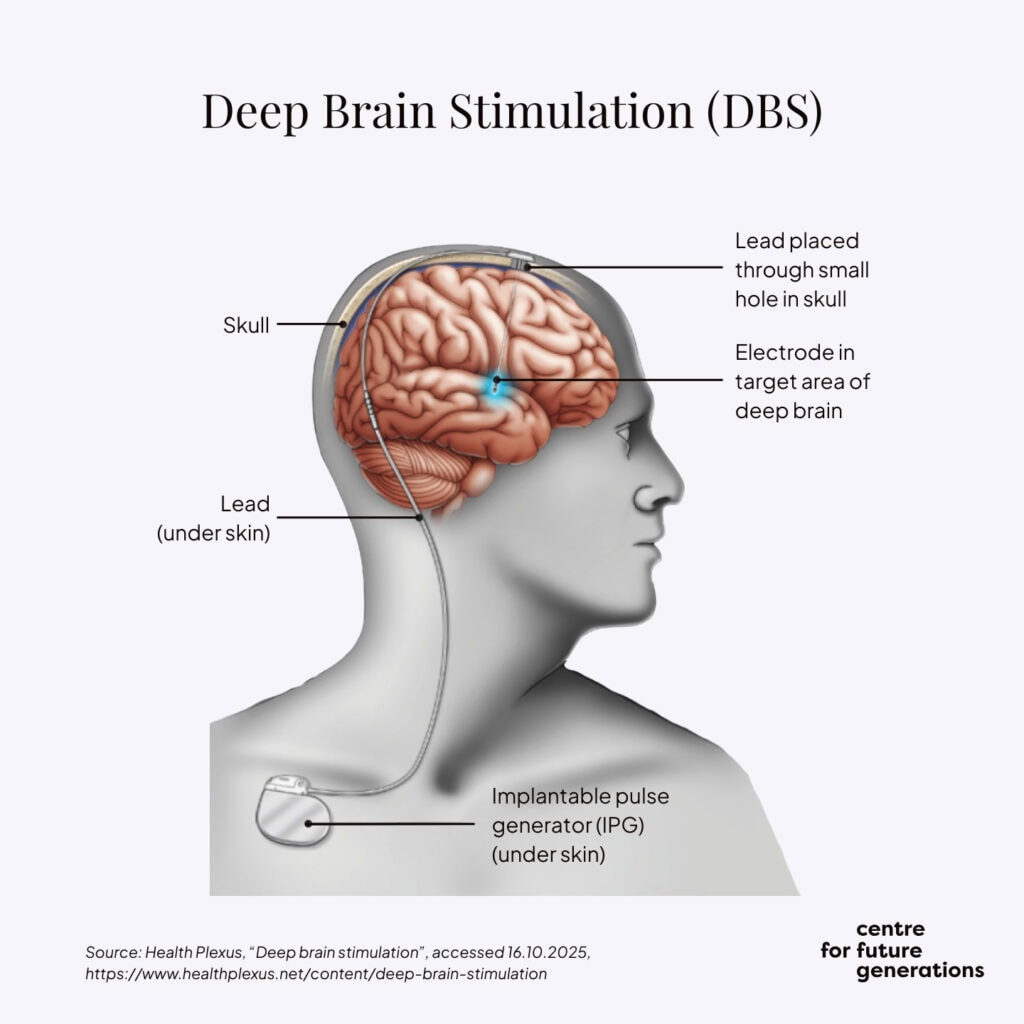
Many of the most impactful neurotechnology innovations in the past 30–40 years have originated in Europe. Pioneering work by Professor Alim-Louis Benabid[6], a French neurosurgeon and researcher, in the late 1980s and early 1990s revolutionised the treatment of neurological disorders by using reversible, adjustable electrical impulses. At Grenoble University Hospital, Benabid and his team showed that neurostimulation of the subthalamic nucleus – a part of the brain involved in motor functions – could significantly alleviate the motor symptoms of Parkinson’s disease. Deep brain stimulation has significantly improved patients’ quality of life without the need for radical surgeries.
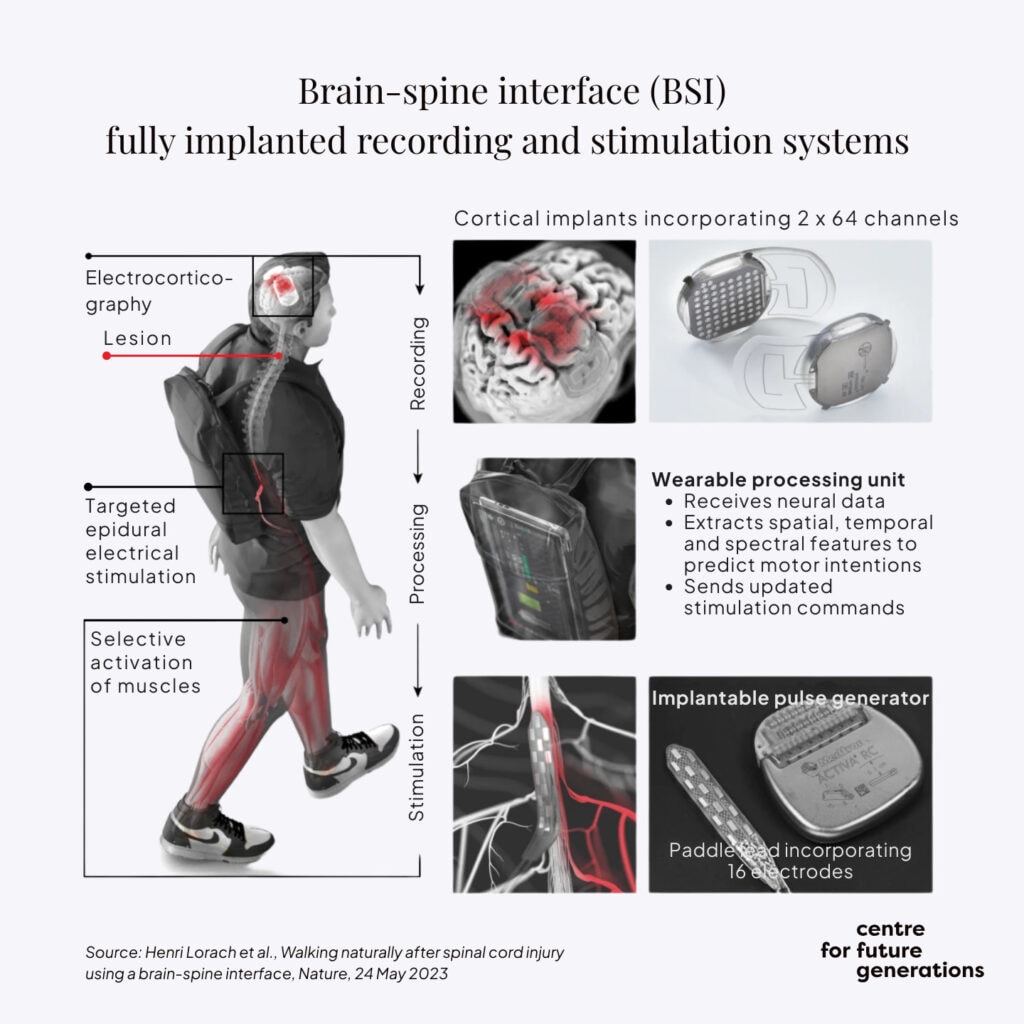
More recently, the team led by Grégoire Courtine at the Swiss Federal Technology Institute of Lausanne developed a brain-spine interface to restore communication between the brain and the region of the spinal cord that produces walking.[7]
Today, the field of neurotechnology is a fast-growing market, in both its medical and, as previous CFG research has illustrated, its consumer dimension[8]. Advances in neurostimulation devices, including adaptive deep-brain stimulation, vagus-nerve stimulation and transcranial magnetic stimulation as well as brain-computer interfaces and AI-enhanced diagnostics, are substantially improving outcomes for patients. The global market in neurotechnology is estimated at $17.3 billion (€15.2 billion) and projected to reach $52.9 billion (€46.3 billion) by 2034, a compound annual growth rate of 13.2%.[9]
Following a well-known pattern, many of these neurotechnology innovations have not been commercialised in Europe, in spite of their scientific roots on the continent. There is now a moment of opportunity to reverse this prevailing trend.
Given Europe’s demographic trends, the demand for effective diagnostic and therapeutic solutions will grow considerably in the next two to three decades. With the necessary investment, the conditions will soon be ripe for Europe to seize the opportunities of neurotechnology for the benefit of its patients, as well as European innovation and competitiveness.
A global co-leadership window the EU cannot afford to miss
Neurotechnology sits at the intersection of biomedicine, AI, digital infrastructure and advanced engineering – all areas of strategic importance for Europe. And, similar to space, quantum and semiconductors, it is a foundational technology where early movers set the standards, capture scarce talent and data, and build large-scale ecosystems.
EU-level engagement is necessary to remain on par with the US and China[10], both of which are investing heavily in the field, including with dual-use technologies in mind. Reassuringly, as the CFG “Neurotech consumer market atlas” has made clear, North America leads the global neurotech market, but Europe is not far behind.
There are ample ways in which the development of neurotechnology aligns with the EU’s quest for strategic autonomy: technologies such as semiconductors are foundational to both the current applications of neurotechnology and future breakthroughs. Brain research is already benefiting from streamlined fast access to computing resources, as underlined in the AI Continent Action Plan.
A dynamic growth of the field can create an important lever of demand for critical technologies which are at the heart of Europe’s quest for greater economic security.
To make Europe’s approach truly strategic, the EU needs to have better clarity about its objectives. As the recently published “Choose Europe for life sciences” strategy of the European Commission spells out, “the EU must identify emerging scientific breakthroughs at an early stage through “horizon scanning” and then support rapid translation into innovation[11]. The EU’s approach to neurotechnology should be guided by four pre-established principles to ensure coherence, efficiency and high-impact outcomes, as outlined below.
Principle 1: Target areas of unmet medical need
Conditions like major depressive disorder, epilepsy, Parkinson’s disease and chronic pain can be resistant to treatment in some patients. One-third to a half of people with major depressive disorders do not respond to multiple antidepressants, while more might show only a partial response.[12] Neurotechnology – including invasive methods like deep brain stimulation and non-invasive techniques, such as transcranial magnetic stimulation and other forms of modulation – offers alternatives where drugs fail[13]. Similarly, as the example of innovative neuroimmune modulation shows, neurotechnology can support patients who are not sufficiently managed, or do not tolerate drugs, in this case biological and synthetic disease-modifying anti-rheumatic ones. This is important, as US studies have shown that only 25% of rheumatoid arthritis patients are satisfied with therapy, and up to 50% discontinue their prescribed therapies within two years, as a result of inadequate or diminishing outcomes or intolerance to adverse effects.
One of the most extensively used neurotechnology approaches, deep brain stimulation, enables the direct measurement of pathological brain activity and the adjustable stimulation of affected circuits. While deep brain stimulation has an established track record in treating Parkinson’s disease, further research is needed to optimise stimulation targets[14] and parameters, improve patient selection, and develop personalised treatment plans to increase both efficacy and safety in other disorders, such as treatment-resistant depression. Crucial questions concern the selection of brain areas to be targeted and patients to be treated, as several factors are of essence, including disabling tremor unresponsive to medication and no significant cognitive or psychiatric comorbidity.
Hardware and software for spinal cord stimulation in pain management have become technologically more advanced than deep brain stimulation thanks to ever-smaller pulse generators.[15] Funding is now needed to bring deep brain stimulation and other similar technologies to the same level. This could result in the development of stimulation on demand or adaptive closed-loop deep brain stimulation with continuous modulation, where deep brain stimulation parameters are selected and modified to reflect the underlying neural circuitry.[16]
Neurotechnology can also be used to guide pharmacological interventions by capturing brain activity in real time. Measuring brain blood flow and oxygenation patterns non-invasively with helmet-like devices offers insights into brain function. This approach can help predict which patients are more likely to benefit from a given treatment and monitor treatment effects in real time, optimising dose or timing.
Principle 2: Prioritise cost-effective solutions to large-scale problems
Far from being a niche area, neurotechnology can provide scalable solutions. One of its most widespread applications, the cochlear implant, has restored hearing in hundreds of thousands of patients, including children, enabling them to participate in a typical classroom. Other such scalable applications are within reach.
Chronic pain is a debilitating condition that affects hundreds of millions of people globally. It significantly diminishes quality of life, limits functional capacity and reduces productivity. In Europe, about 150 million people experience chronic pain, defined as pain that persists for more than three months; neuropathic pain, a type of chronic pain that results from nerve damage, affects 7–8% of the population.[17] Despite its prevalence and substantial personal and societal burden, chronic pain remains consistently underdiagnosed and undertreated. Many health systems lack structured pathways for the early identification, comprehensive assessment and multidisciplinary management of chronic pain. As a result, millions of people continue to live with unrelieved symptoms, often leading to mental health comorbidities, reduced employment, and increased pressure on healthcare services.
Spinal cord stimulation is a treatment option for certain chronic pain conditions, particularly neuropathic pain. It involves implanting a device that delivers electrical pulses to the spinal cord, aiming to disrupt pain signals. Spinal cord stimulation has demonstrated efficacy in managing a growing number of conditions. Analyses have shown that among patients who continued spinal cord stimulation for at least two years, a significant proportion could reduce or discontinue their systemic use of opioids, with greatly reduced costs after the start of the therapy.[18] Yet despite its potential benefits, only about 5% of patients who are candidates for these neuromodulation therapies receive them, because of limited access to specialised care, a lack of awareness or funding constraints.[19]
Rewiring of the brain after stroke is another potentially impactful area of intervention. Neural stimulation is now investigated as a method to enhance connectivity during rehabilitation and assist the brain in relearning how to use arms after stroke. If successful, this approach could help stroke patients significantly improve the quality of their lives.
The ongoing trend towards the development of wireless and minimally invasive solutions, including spinal cord stimulation, allows for the prospect of scaling neurotechnology devices. Wireless spinal cord stimulation systems remove or reduce the need for implanted pulse generators and extensive lead wiring through the body. This means that a paradigm shift is needed in the way neurotechnology is perceived. Far from addressing only a narrow range of conditions, the approach can be applied to a wide spectrum of neurological and mental disorders, the number of which is growing significantly.
Principle 3: Double down on areas of high impact in severe disability
Some neurotechnology solutions, particularly brain-computer interfaces, hold the promise of relieving some of the most severe disabilities known to humankind, such as myotrophic lateral sclerosis or locked-in syndrome. The former is a progressive neurodegenerative disease which leads to muscle weakness and atrophy, translating into increasing difficulty of performing daily tasks. Patients with locked-in syndrome, while retaining cognitive function and awareness, face profound barriers to self-expression and mobility. Establishing a functional communication system is an important part of increasing or maintaining patients’ quality of life. Because of the reduced muscle activity in patients with locked-in syndrome, traditional augmentative and alternative communication methods may be insufficient.
Brain-computer interfaces and neural prosthesis are emerging as techniques to restore these patients’ autonomy and life quality. In their non-invasive form, brain-computer interfaces are characterised by reduced risks but bring more challenges related to signal detection and interpretation. Invasive brain-computer interfaces establish a more direct connection with neural networks and lead to more reliable communication and control. BCIs are being tested in neurosurgery, where tools such as electrocorticography (ECoG) may help reduce risks during epilepsy and tumour resections by detecting and preventing intraoperative seizures. These technologies are rapidly improving and show huge potential, but require further research to make them more accessible and effective.[20]
Principle 4: Pivot towards prevention, detection and monitoring
To unlock the full potential of neurotechnology, encompassing bioelectronic therapy, a forward-looking strategy should prioritise the development of tools and platforms that enable early detection, monitoring and prevention of brain disorders across the human lifespan. Strategic investment in this area would accelerate the transition from reactive treatment models to proactive, preventive approaches, particularly for chronic and progressive neurological conditions such as Alzheimer’s disease, mental-health disorders and stroke. Advancing early-stage interventions not only improves patient outcomes but also has the potential to significantly reduce long-term healthcare costs and societal burdens.
One illustration of this phenomenon can be found in the prodromal phase of dementia. Epidemiological studies show that about 35-50% of individuals with mild cognitive impairment will experience cognitive decline towards dementia within 3-5 years[20a]. Yet current clinical investigations of mild cognitive impairment are costly, resource-intensive, and time-consuming, while offering only limited predictive power regarding disease progression. For prevention to be effective, however, heterogenous risk factors and comorbidities, including endocrine-metabolic disorders, cardiovascular disease, diabetes, diet and sedentary lifestyle, and sensory impairments need to be fully taken into account. As disease-modifying treatments begin to emerge, the need for efficient and reliable screening methods for early diagnosis becomes pressing. The EU-funded AI-Mind project, has taken an important step in this direction by developing novel tools to accelerate the diagnostic process and assess an indvidual’s risk of developing dementia[20b] These combine high-density magneto- and electroencephalography recordings with computerised cognitive tests, genetic and protein biomarkers, as well as sociodemographic and clinical variables. Looking ahead, such multimodal approaches should be further enriched with behavioural data new generations of sensors and consumer neurotechnology, enabling far greater predictive accuracy and integration into routine healthcare practice.
Wearable neurotechnology devices, including neuroimaging tools and brain-computer interfaces, offer unique opportunities to gather real-time, high-resolution insights into brain function and dysfunction well before symptoms emerge. Given the non-invasive nature of these devices, they are more easily integrated into everyday life and can hence involve much larger groups of users. By harnessing the resulting data streams, funding can support studies and applications grounded in a mechanistic understanding of biological, psychosocial and socioeconomic risk and protective factors.
Funding should also aim to draw insights from the cumulative effects of modifiable lifestyle and environmental influences. This includes exploring digital and psychosocial interventions that promote brain health, foster behavioural change and reduce stigma through public engagement and inclusive communication. Prioritising neurotechnology innovation in prevention can help reduce the long-term burdens of disease and build a new foundation for brain-health policy and practice.
Alongside increased funding, it is essential to address the current policy challenge that arises from differing regulatory regimes for consumer products and medical devices. The category of borderline devices, which straddles these two areas, should have its own distinct framework to prevent innovation from becoming siloed.[21]
Recommendations
Strategic areas of research
As the “Choose Europe for life sciences” strategy emphasises, “the creation of new knowledge is an essential basis for a vibrant life sciences ecosystem and for the development of technologies and innovations”[22]. To deliver on their significant potential, advances in neurotechnology require further scientific progress in several disciplines.
- Core neuroscience. Current neurotechnology approaches rely on an incomplete understanding of how complex brain functions, such as memory, emotion, consciousness and creativity, emerge from neural activity. Future research needs to provide deeper insights into connectomics – the science of neural connections -to disentangle how brain regions interact, explore models of neuroplasticity and learning mechanisms, and offer a greater understanding of non-neuronal cells and their function. Much of this study should be underpinned by improved high-resolution brain mapping, both spatial and temporal.In addition, connecting the different levels of brain organisation -from molecules and cells to circuits and behaviour – is of the essence. Neurological and mental disorders often arise from dysfunctions that span multiple levels, from genetic mutations (molecules) and synaptic disruptions (cells) to network imbalances (circuits).
- Advanced materials. In parallel with advances in neuroscience, research is needed in materials science and bioengineering, since brain implants’ interaction with brain tissue is still limited by inflammation, degradation and poor integration. There is scope for development of soft, flexible bioelectronics and neuro-compatible materials that can blend with brain tissue.
Currently used metals, such as platinum and iridium, cannot guarantee long-term durability inside the human body in bidirectional mode required. This is why the European start-up INBRAIN Electronics is harnessing the potential of graphene, which enables novel, flexible, high-resolution, high-precision neurotechnology.
New materials are also needed in batteries, where miniaturisation and flexibility are key. Ultra-thin, biocompatible batteries can be more easily integrated into flexible implants. In a future “battery free” implant paradigm, energy can be delivered wirelessly on demand. Hybrid systems that use small onboard reserve batteries with wireless charging for peak demands.
Bio-inspired approaches, merging soft materials with tissue engineering to produce “living interfaces” are also an important field of exploration. They can result in enhanced integration and improved durability.
- Compute and AI. Reading, analysing and interpreting brain data requires massive computational power and sophisticated models. To achieve this, neural, behavioural and psychological data must be integrated. Furthermore, the use of virtual twins for simulation and prediction in treatment planning would significantly improve the performance of personalised diagnostic and therapeutic tools.
Such projects should be treated as part of a strategic European Initiative to be granted streamlined access to EuroHPC supercomputers, without the requirement of submitting to a Peer-Review process.
- Precision engineering. Several areas require combined scientific and technical progress. Existing approaches, such as transcranial magnetic stimulation or deep brain stimulation, in spite of impressive progress, continue to lack sufficient target specificity and personalisation. As a result, techniques for cell-type-specific stimulation need to be developed. Closed-loop systems that respond in real time to brain activity are required, as are personalised stimulation protocols based on patient biomarkers. All of this needs to be anchored in a better understanding of the long-term effects on neural circuits. Recent innovation in this area includes NaviNIBS – Neuronavigated Noninvasive Brain Stimulation platform which improve targeting accuracy.
Improvements are also necessary in brain-computer interfaces, many of which are invasive, have low bandwidth or are not adaptive to natural brain rhythms. The emergence of non-invasive or minimally invasive brain-computer interfaces[23], where devices are delivered endovascularly, is opening a new phase in the scaling of neurotechnology, particularly when their wearability, reliability and biocompatibility improve.
Stereotactic robotic arms are already being used for highly accurate placement of electrodes in DBS and neurorobotics platforms link real-time imaging with robotic navigation to guide resection and further research is needed to move ECoG-and BCI-guided neurorobotics from concept towards clinical integration.
The area is attracting significant interest around the world: UK Advanced Research and Invention Agency’s (ARIA) Precision Neurotechnologies programme aims to unlock new methods to interface with the human brain at the circuit level[24].
In an ambitious bet at the intersection of neuroscience and consumer technology, Nudge’s non-invasive brain interface is a headset that can both stimulate and image the brain while Forest Neurotech is working on a whole-Brain Computer Interface (wBCI) enabling both imaging and neuromodulation over large volumes of the human brain.
- Ethics. Continued research is also needed in the field of responsible research and innovation, as neurotechnology touches on fundamental issues of privacy, agency, mental autonomy, human identity, consent and equity. Cognitive enhancement and mental state shifts through wearable devices are stated objectives of an increasing number of companies active in the neurotechnology space. As an example, in its mission statement, Nudge says: “In the longer-term, we see the technology being used by everyone for applications as wide-ranging as improving mood, focus, and memory”.
Anticipatory frameworks for ethical use of brain data must be developed and constantly updated. Further studies on the psychological and social impacts of neuro intervention are needed, as are legal and ethical definitions of mental integrity, autonomy and identity.
Ethical gaps are particularly pronounced in closed-loop neurotechnology which adapts to patients’ neural states with real-time feedback mechanisms.Building on the European Charter for the Responsible Development of Neurotechnologies, devised by a task force led by the European Brain Council, the EU has an opportunity to set global standards for ethical neurotechnology[25].
Propelling innovation from the lab to the market
Although there are some important recent regulatory approvals of neurotechnology solutions, many projects are in the research or pilot phase, given the continuously emerging nature of the field. To ensure their clinical translation and scaling, they need standardised measures of outcomes, robust clinical validation and cross-disciplinary trial frameworks. EU-level funding can support the creation of common standards and interoperable platforms.
Neurotechnology is one of the four focus areas of the EU’s Testing and Experimentation Facility for Health AI and Robotics (TEF-Health) initiative, which aims to accelerate the development, testing and certification of trustworthy AI and robotics solutions in health care by providing a network of physical and virtual testing environments across Europe. By enabling rigorous testing in real-world clinical settings, TEF-Health helps ensure that neurotechnologies meet high standards of safety, efficacy and ethical compliance. The EU should further develop neurotechnology-specific translational platforms to allow for clinical-grade testing environments.
In addition, the EU will need to develop a robust health technology assessment (HTA) framework for validation purposes in neurotechnology. Under the EU HTA Regulation, Joint Clinical Assessments (JCAs) will be required for certain high-risk medical devices, potentially including neurotechnology devices, provided they meet specific criteria, including addressing unmet medical needs, first-in-class innovation, public health impact, use of AI/software, cross-border relevance, and EU-wide added value. This means that companies can already request guidance on clinical development from the HTA Coordination Group (involving Member State experts), with JCAs becoming mandatory from 2026 but focusing strictly on clinical safety and performance, not cost-effectiveness, given that pricing decisions remain at the Member State level.
Ethical and data-governance issues and the use of responsible AI need to be at the heart of the innovation framework, to ensure compliance with existing EU policies and ethical standards and oversee the development of new ones. Since data is the foundation of innovation in neurotechnology, data-sharing infrastructures are needed to bring progress to scale. While the European Health Data Space (EHDS) Pilot has not included a use case in the area of brain health, EBRAINS – the research infrastructure for brain studies has been part of the pilot and aims to become a Node of the EHDS infrastructure, ensuring functional access to brain data. Data-analysis tools offered by EBRAINS and other Research Infrastructures will enable a better understanding and mapping of brain functions, contributing to personalised medical approaches.
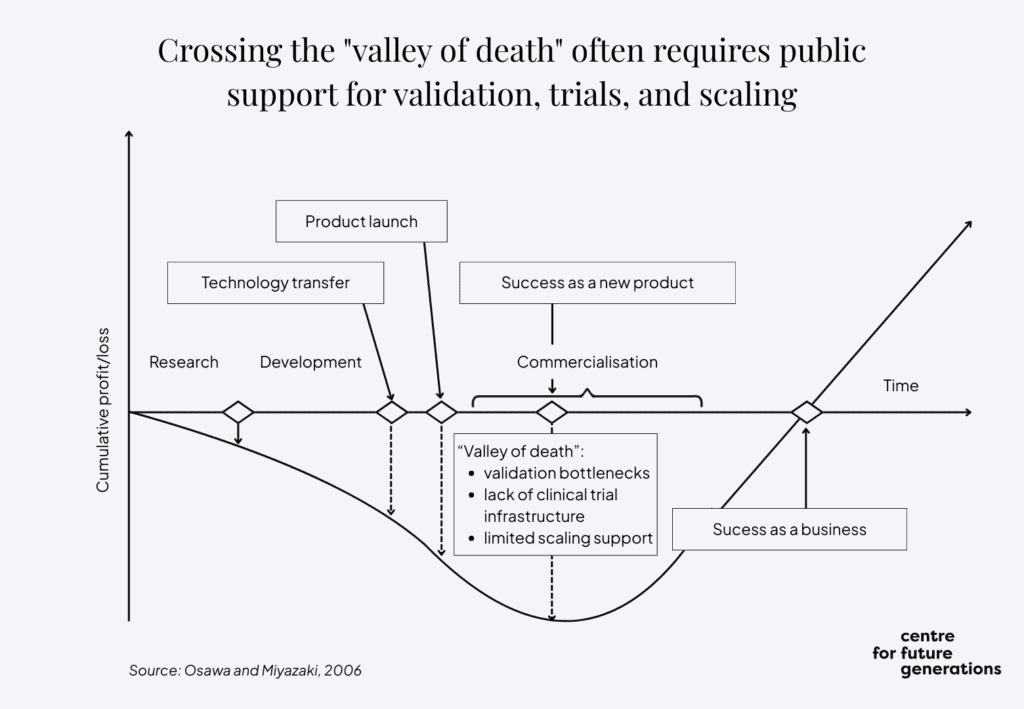
In this context, the EU should develop multiphase funding models that span discovery, prototypes and clinical trials. This means providing seed funding and incubation pathways in the initial period, followed by direct and indirect funding in later stages. Dedicated support in the area of intellectual property and tech transfer would play an important supporting role, given the specificities of the field.
A public private catalytic funding approach can play a critical role in derisking early-stage innovation and progressively steering private investment – from venture capital, private equity, corporate actors and financial institutions – to take over as public support tapers off. This handover would ensure a sustainable innovation pipeline that can bridge the gap between research and market-ready neurotechnology solutions. Multiphase funding would help overcome the neurotechnology version of the valley of death between initial R&D and market take-up. This approach would be in line with the Flagship initiative of the “Choose Europe for life sciences” strategy, which proposes launching a matchmaking strategic interface connecting life science startups, industry and investors.
At the regulatory level, neurotechnology would clearly benefit from streamlining of procedures for authorisation of medical devices on the EU market, for instance by means of a centralised authorisation body as proposed by MedTech Europe.
What being “Wired for the future” means for the EU
Neurotechnology is at the cusp of rapid acceleration, enabling real-time healing of the nervous system. A strategic approach to the field, linking actors across the life-cycle of innovation, pulling funding and aggregating demand, can propel the EU to the heart of this new area of global competition. Building on an impressive level of scientific achievement and technological expertise, neurotechnology in the EU needs its own moonshot effort.
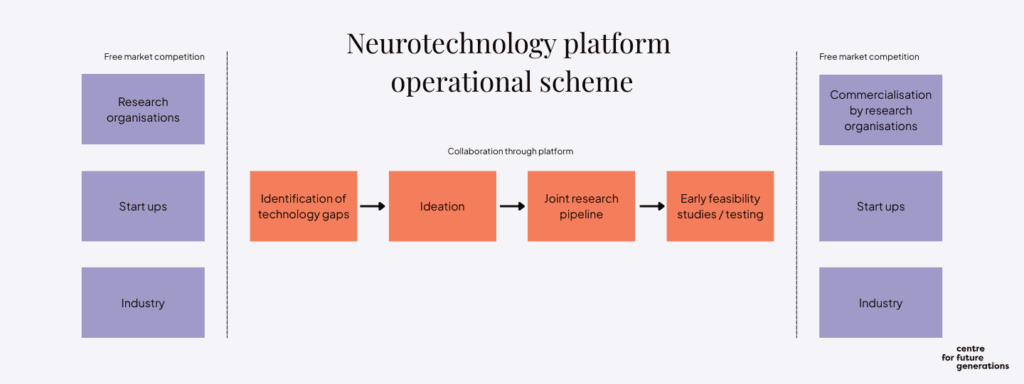
→ An EU Neurotechnology Medicine Platform needs to be created to stepchange pre-market collaboration
Neurotechnology is one of the most interdisciplinary areas in science and innovation. It draws on advances in brain research and medicine as well as compact electronics and powerful computation. The next generation of the technology must enable a more integrated and scalable approach, with broad applications across health, cognition and human performance. It must be based on new advanced materials, enabling high-fidelity neural recordings, better biocompatibility, more flexibility and thinness, as well as durability.
Pre-competitive collaboration will pave the way to market success
While the EU has developed successful models for Research and Innovation ecosystems, which include partnerships, missions and life sciences clusters, it is missing a critical element of effective pre-competitive collaboration. To this end, the EU should launch a platform focused specifically on the phase before companies compete directly on the market, to accelerate the development of core technologies that can support therapeutic innovations. Such technologies include high-resolution interfaces, data-integration frameworks and adaptive algorithms and would focus on conditions like Alzheimer’s disease, other dementias, blindness and stroke. By focusing on shared infrastructure and interoperable building blocks, a precompetitive platform would reduce duplication, lower barriers to entry and catalyse innovation across the neurotechnology ecosystem.
Drawing on well-tested European and international models
Similar efforts have been successfully launched in other fields of science. The European Molecular Biology Laboratory (EMBL), which is formally an intergovernmental organisation – like CERN, but for life sciences. The EMBL provides pre-competitive services in data, technologies, and training that lower barriers to innovation. It has pioneered open data resources, such as Ensembl, UniProt, and the European Nucleotide Archive, which have become indispensable to global life sciences. Its governance model demonstrates the value of a neutral, intergovernmental platform trusted by academia, industry, and regulators alike. While EMBL does not develop commercial products, its outputs are widely adopted – precisely the kind of enabling ecosystem that is also needed for neurotechnology.
When it comes to sharing data and samples in neurodegenerative disease research, comparable features and governance are displayed by the European Platform for Neurodegenerative Diseases.
In the US context, the National Institutes of Health’s programme focused on Stimulating Peripheral Activity to Relieve Conditions (SPARC) is a close analogue. Focused on developing therapeutic solutions that modulate electrical activity in nerves to improve organ function, it is explicitly set up to lower barriers for both academia and industry by creating common resources. SPARC was established to accelerate the field of bioelectronic medicine by creating a pre-competitive ecosystem of data, tools, and knowledge. The programme’s SPARC Data and Resource Center curates high-resolution anatomical and functional datasets and makes them openly accessible under FAIR principles. The SPARC Portal platform promotes data reuse, collaboration, and translation.
The initiative should be anchored by a well-established actor in European life sciences
The EU Neurotechnology Medicine Platform (EU-NMP) would aim to catalyse innovation at the precompetitive stage by accelerating the emergence of new scientific fields and enabling future commercial medical applications.
The platform could be developed by one of the existing undertakings in the EU ecosystem, including the Innovative Health Initiative or the European Institute of Innovation and Technology (EIT) Community in health, the EIT-Health, which spans education, entrepreneurship, investment, and cross-sector collaboration. In the process, it could interface with existing life sciences clusters, which bring together different stakeholders to accelerate innovation.
This platform would require a new organisational structure that breaks down traditional boundaries and fosters sustained collaboration across sectors. This model must bring together the scientific creativity and exploratory mindset of academia, the engineering scale, talent and executional discipline of industry, and the clinical expertise and patient-centred perspective of health-care providers. Such a structure would not only accelerate the development of transformative technologies but also ensure that these innovations are relevant, safe and deployable in real-world health systems.
Making the most of both academic and industrial ecosystems
Academic environments are essential incubators of bold ideas but often lack the resources and infrastructure to translate discoveries into scalable solutions. There are typically few incentives for scientists to dedicate time and effort to translation. Conversely, industry offers the engineering capabilities and operational rigour needed to build, test and deploy technologies at scale but may not always have the proximity to frontier science or patient needs. Clinicians, meanwhile, bring a deep understanding of disease complexity, comorbidity and patient experience, which is crucial for aligning innovation with health outcomes.
An integrated organisational approach that brings together elements of public-private partnerships, mission-driven hubs or consortium models can bridge these capability challenges. It can foster shared governance, rapid iteration and co-development pathways that allow ideas to move from bench to bedside more efficiently. To be effective, such a model must also embed ethical oversight, equity considerations and mechanisms for inclusive stakeholder engagement to ensure that technological progress translates into meaningful societal benefit.
Modularity in the focus to make everyone benefit
The platform would be a modular, interoperable arena in which companies could develop components such as high channel-count neural interfaces, device powering systems, wireless communication modules and other technologies and configure them to treat a range of neurological conditions. A modular approach could help reduce redundancy and cost. Its requirements-driven logic would ensure that the focus is squarely placed on areas of innovation where biggest challenges persist and where collaboration in the pre-market phase would translate into better outcomes.
A core priority of the EU-NMP would be to support early feasibility studies in neurotechnology medicine, similar in spirit to Phase 0 trials in the pharmaceutical sector. This approach would help derisk breakthrough technologies before large-scale investment while generating early clinical insights.
All technologies developed on the EU-NMP would undergo rigorous testing for safety, clinical operability and efficacy to ensure that their modular combinations had a regulatory and translational advantage in future use. By building a foundation of validated, adaptable components, the EU-NMP would significantly reduce the time it takes a company to get a treatment into clinics. The platform would also include an R&D pipeline for the participating organisations and establish a manufacturing function to deliver small volumes of components. These components would undergo regulatory tests and be disseminated to leading European neuro-translational groups. The results would catalyse innovation with the aim of achieving clinical solutions on a broad scale.
Holding the bar high in regulatory innovation and multistakeholder engagement
Going beyond collaboration focused on research and innovation, the pre-competitive platform should have a regulatory sandbox function. In this way, it would support the emergence of a legal framework which is best suited for the needs of neurotechnology. It would also help to assist medtech companies in meeting regulatory compliance requirements.
A foundational principle of the EU-NMP should be multistakeholder engagement, which would ideally lead to a Multistakeholder Engagement Charter, a commitment to integrate the input of all relevant actors. The platform would need to rely on the science of patient-citizen input, which is critical to unmask early signs of disease. It should also take advantage of previous innovative participatory and anticipatory governance models, such as that of the EU-funded MULTI-ACT project, which aimed to increase the impact of health research on people with brain diseases.[27]
→ A dedicated Neurotechnology Funding Board is needed to align and strengthen financial instruments, while creating new ones where gaps exist
EU funding for research and innovation has traditionally followed a generic approach in which picking winners is avoided and projects compete based on excellence. In fundamental science, the policy of keeping a balance between disciplines has prevailed in funding approaches of the European Research Council (ERC). The ERC’s funding model follows a bottom-up approach, meaning it does not set predefined thematic or disciplinary priorities. Its main grants, and other Principal Investigator-led actions, are evaluated on the sole criterion of excellence. Horizontal calls dominate other parts of the Horizon Europe programme, with thematic clusters and missions addressing broad societal challenges rather than narrow fields.
Reflecting neurotechnology’s specificity without funding carve-outs
Neurotechnology, with its medical importance and interdisciplinary nature, calls for a middle ground between broad, generic funding approaches and narrowly domain-specific ones. While it can and should benefit from multiple funding streams, reflecting the diverse technological developments it builds on, it also has distinct integrative requirements. These demand deep insight into the functioning of the neural system and therefore cannot be left solely to the horizontal, cross-cutting initiatives. Some tailor-made solutions are needed to ensure coherence and impact.
Neurotechnology Funding Board should optimise investments
Stepping up investment in neurotechnology should take place across the EU funding landscape. For this purpose, the European Commission should create a Neurotechnology Funding Board to oversee and feed into the development of funding streams across current and future instruments. The board would aim to maintain cohesion between these instruments and ensure adequate funding flows to them. It could be seen as a subset of the Life Sciences Coordination Group proposed by the “Choose Europe for life sciences” strategy of 2 July 2025, and afforded a central role in mapping overall opportunities in life sciences, aligning funding priorities and integrating ongoing activities.
The Neurotechnology Funding Board would:
- Closely monitor generic funding streams and incorporate criteria relevant for neurotechnology.
- Inform the neurotechnology research and innovation community comprehensively about emerging opportunities in generic fields and prepare guidelines for applicants.
- Formulate a long-term research and innovation agenda together with the community.
- Define a range of domain-specific funding opportunities across the EU’s portfolio of instruments.
The board would focus primarily on various EU instruments, such as Horizon Europe and its Digital Europe programme, but also on partnerships, including the Innovative Health Initiative (IHI), as well as the newly proposed instruments such as the Scaleup Europe Fund, announced in the European startups and scaleups strategy, which recognises the strategic importance of life sciences and biotech. The latter is aimed at bridging the funding gap, and overcoming the “valley of death” challenge, relating to the high-risk phase between early development and commercial deployment.
Active sourcing of funding ideas needed and infusion of specialist knowledge
The board would have two primary areas of activity. First, it would propose new domain-specific funding calls, having solicited input from the neurotechnology research and innovation community through a channel similar to the IHI Ideas Incubator. Second, it would be involved in the elaboration of the horizontal funding calls, making them as relevant as possible to the neurotechnology community. It would communicate actively with a pool of neurotechnology research groups and companies to make sure they are aware of both the domain-specific and generic calls. The board would also collaborate with the EU Member States on the setting of national funding priorities.
Apart from drawing on existing and emerging funding instruments, the EU should develop new investment frameworks to reflect the unique challenges and opportunities of this rapidly evolving field. This should include a Neurotechnology Joint Undertaking, bringing together both private and public-sector partners. In addition, sufficient space should be created for hybrid operational models that combine for-profit and not-for-profit approaches and bridge public, philanthropic and private capital while embedding patient involvement, clinical validation and regulatory foresight from the outset. An optimised ecosystem would integrate investment, solution testing, user engagement and impact measurement into a cohesive process to ensure that promising technologies move efficiently from early development to real-world application.
Integrate neurotechnology among the new European “Moonshot” projects
Given neurotechnology’s meteoric rise in terms of breakthrough solutions and recently announced capital investments, the field should be included among the new “moonshot” projects that the European Commission envisages for the 2028-2035 funding cycle in the new Multiannual Financial Framework. Neurotechnology meets the criterion of having a strong scientific component and being able to boost EU-wide value creation and strategic autonomy. One of the possible options would be to integrate neurotechnology with the proposed moonshot focused on innovative therapies for human regeneration.
→ Aggregate demand by developing neurotechnology-specific reimbursement models
Given their nascent status, current neurotechnology solutions remain difficult to access and expensive. Their cost reflects the complexity, risk and novelty of the technologies involved. Prices can vary significantly depending on the type of solution, its intended use and the stage of adoption. Devices in areas such as deep brain stimulation, brain-computer interface and neuroprosthetics require precision-engineered components, which are often customised. Materials must be biocompatible and durable, driving up costs.
Make reimbursement a critical catalyst of adoption
In the EU, neurotechnology solutions are increasingly recognised as bringing clinical benefit to be eligible for reimbursement. Several neuromodulation devices (e.g. deep brain stimulation for Parkinson’s, spinal cord stimulation for chronic pain, vagus nerve stimulation for epilepsy and depression) are already reimbursed in many EU Member States.
Yet, reimbursement decisions are not strongly co-ordinated, and are occasionally reversed. In this context, the EU has a compelling need to develop neurotechnology-specific models to address the current fragmentation of HTA frameworks and the absence of common criteria in pricing and reimbursement policies across Europe.
To support the adoption of innovative neurotechnology, the EU should design and test dynamic pricing mechanisms and align these with real-world evidence, which can help close the gaps in the evaluation process. In addition, it should explore value-based procurement and outcome-based funding to ease cost burdens and reward efficacy. In fact, a dedicated European neurotechnology procurement pathway could take the form of a uniform procedure, where EU-level spending is matched with the national one.
A subscription model could also be used, given its particular applicability to disruptive solutions and technologies. Practiced in countries such as the UK to treat infections caused by drug-resistant bacteria, this new payment model, dubbed “Netflix for antimicrobials”, aims to incentivise development of new antibiotics without their overuse. Under the Antimicrobial Products Subscription Model, NHS England pays a fixed annual subscription fee to a pharmaceutical company for guaranteed access to new products. A similar subscription model could be used for purchase of targeted neurotechnology solutions.
An avantgarde of Member States can make a big difference
Scaling neurotechnology across the continent, in the interest of both innovation as well affordability and better access, would be significantly enhanced if a group of Member States agreed to align and synchronise their reimbursement decisions, pooling resources and hence creating a significant pull factor for the emergence of neurotechnology solutions. Member States should recognise the value of strengthening their future purchasing power. They should also draw conclusions from vaccine development where advanced public commitments made an enormous difference. An analogous approach would require an avantgarde of Member States to set end-point objectives and encourage neurotechnology developments that satisfy stated requirements.
Conclusions: A novel approach to harnessing neurotech in Europe
Neurotechnology is a major area of innovation with a significant potential to impact related scientific and technological domains, given its interdisciplinary nature. It is also increasingly recognised as an indispensable part of brain health, given the complexity and intractability of brain disorders, many of which cannot be addressed through pharmacological approaches. Europe is uniquely placed for a leading role in this field, but it needs to build a competitive edge given intensifying efforts of other international players.
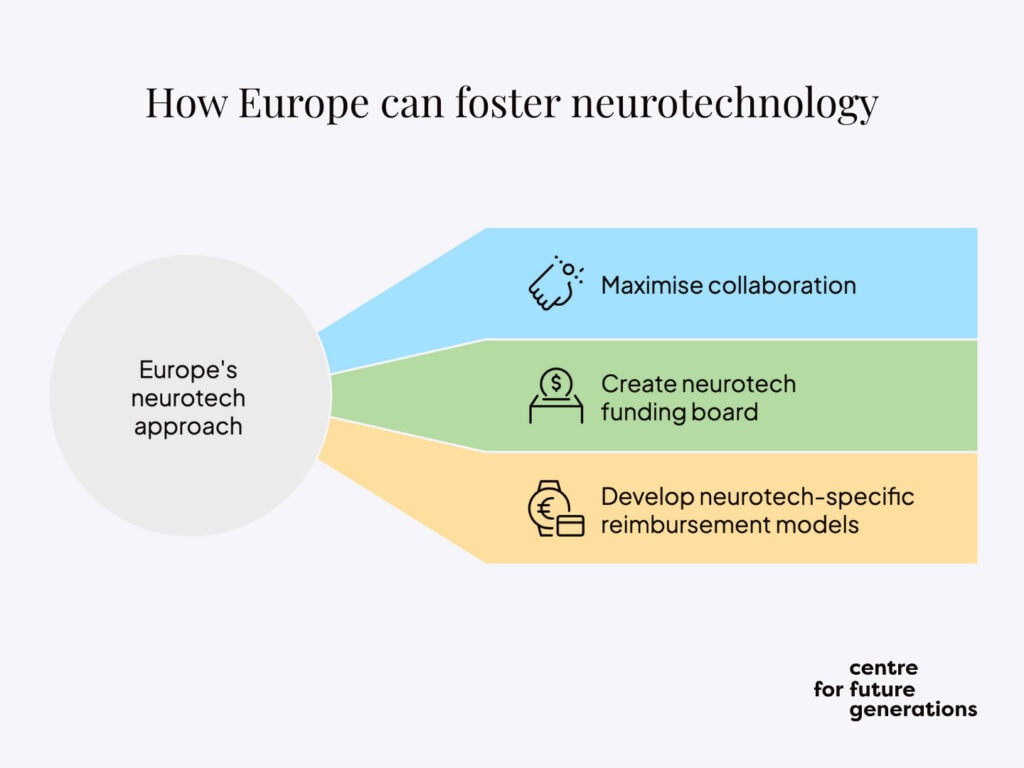
To do so, Europe should adopt a three-pronged approach. First, it should maximise pre-competitive collaboration to ensure that synergies are built between the different research efforts, with view to optimising innovation opportunities further downstream. Second, it needs to create the Neurotechnology Funding Board to strategically adapt existing funding workstreams and identify new ones to fully realise an ambitious research and innovation agenda. Third, it should develop neurotechnology-specific reimbursement models and pool resources among committed Member States to provide the basis for a significant up-take of neurotechnology in Europe’s health systems.
Progress in neurotechnology is an excellent example of technological convergence, as the integration of AI and machine learning enhances capabilities in brain-signal analysis, leading to more effective diagnostics and treatments. By supporting the emergence of neurotechnology, the EU can create a new major area of innovation, while contributing significantly to its strategic autonomy.
Endnotes
[1] OECD, Promoting good mental health in children and young adults, 2025, p. 8-9.
[2] European Commission. Brain research.
[3] Deuschl, G. et al. The burden of neurological diseases in Europe: an analysis for the Global Burden of Disease Study 2017. Lancet Public Health 5, e551–e567 (2020).
[4] European Brain Council. Brain Mission (2018).
[5] Fraifeld, E.M. et al. Systemic Opioid Prescribing Patterns and Total Cost of Care in Patients Initiating Spinal Cord Stimulation Therapy: A Retrospective Analysis. Pain Medicine 22, 784–799 (2021).
[6] European Patent Office, Alim-Louis Benabid, Treatment for Parkinson’s disease, Winner of the European Inventor Award 2016.
[7] Lorach, H. et al. Walking naturally after spinal cord injury using a brain-spine interface. Nature 618, 126–133 (2023).
[8] Bernáez Timón L., Mahieu V., Neurotech consumer market atlas, Centre for Future Generations, 2025.
[9] Gokhale, S. Neurotechnology Market Size, Share, and Trends 2025 to 2034. Precedence Research, last updated 16 January 2025.
[10] China’s Neuro-Interventional Device Market Outlook Report 2025-2032, GlobeNewswire, 7 July 2025.
[11] European Commission, Choose Europe for life sciences (2025), p. 11.
[12] Marwaha, S. et al. Novel and emerging treatments for major depression. The Lancet 401, 141–153 (2023).
[13] Lozano, A.M., Lipsman, N., Bergman, H. et al. Deep brain stimulation: current challenges and future directions. Nature Review Neurology 15, 148-160 (2019)
[14] Lozano, A.M., et al., Predicting optimal deep brain stimulation parameters for Parkinson’s disease using functional MRI and machine learning, Nature Communications, 12, Article number: 3043 (2021).
[15] Deer, T. et al. Prospective, Multicenter, Randomized, Double-Blinded, Partial Crossover Study to Assess the Safety and Efficacy of the Novel Neuromodulation System in the Treatment of Patients With Chronic Pain of Peripheral Nerve Origin. Neuromodulation 19, 91–100 (2016).
[16] Lozano, A.M. et al. Deep brain stimulation: current challenges and future directions. Nature Reviews Neurology 15, 148–160 (2019).
[17] European Pain Federation. Pain and Mental Health in Europe (2023).
[18] Fraifeld et al. Opioid Prescribing Patterns.
[19] Lo Blanco, G. et al. Neuromodulation in chronic pain management: addressing persistent doubts in spinal cord stimulation. Journal of Anesthesia, Analgesia and Critical Care 5 (2025).
[20] Voity, K. et al. Update on How to Approach a Patient with Locked-In Syndrome and Their Communication Ability. Brain Sciences 14, 92 (2024).
[20a] Roberts, R.O., et al., Higher risk of progression to dementia in mild cognitive impairment cases who revert to normal. Neurology 82, 317-325.
[20b] Ira H. Haraldsen, et al. Intelligent digital tools for screening of brain connectivity and dementia risk estimation in people affected by mild cognitive impairment: the AI-Mind clinical study protocol, Frontiers in Neurorobotics, 5 January 2024.
[21] This point will be elaborated in a forthcoming CFG publication.
[22] European Commission, Choose Europe for life sciences (2025), p. 7.
[23] Synchron launches patient registry to prepare for brain implant trial, Medtechdive, 9 April 2024.
[24] Advanced Research and Invention Agency. Precision Neurotechnologies.
[25] European Brain Council. European Charter for the Responsible Development of Neurotechnologies.
[26] See Art. 7 of the EU HTA Regulation, https://eur-lex.europa.eu/eli/reg/2021/2282/oj
[27] MULTI-ACT. More impact of health research on people with brain diseases. https://www.multiact.eu/
[28] European Commission, EU Startup and Scaleup Strategy (2025).
[29] Innovation Health Initiative. Propose ideas.





